10 Classified How To Find Electron Latest
8 Exclusive How To Find Electron - What is 2nd ionization energy? In the first example, the number of electrons in kno3 equals (19 x 1) + (7 x 1) + (8 x.
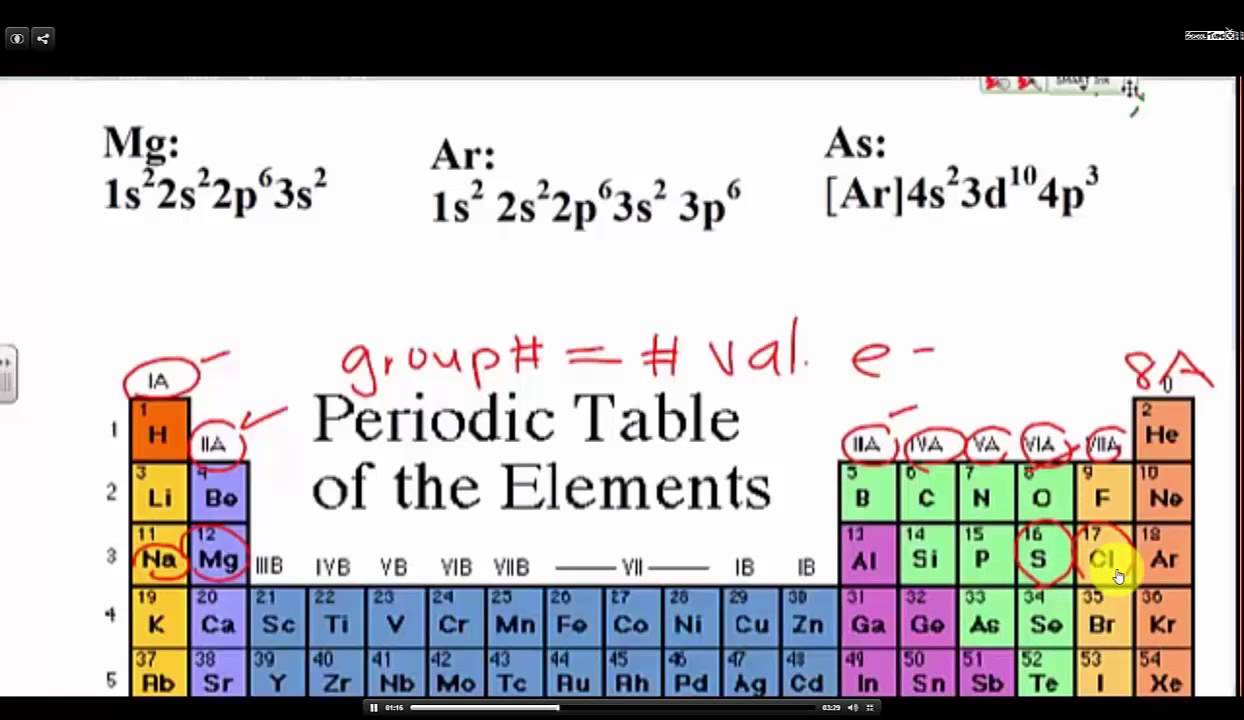 Determine the number of valence electrons from the . It is simple to know the number of protons, electrons, and neutrons by using the periodic table.
Determine the number of valence electrons from the . It is simple to know the number of protons, electrons, and neutrons by using the periodic table.
How to find electron

13 Quickly How To Find Electron. To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. How do you find the number of unpaired electrons? To solve without a periodic table, find the electron configuration of the element and count the electrons into 1 group of 2, and then into shells of 8. How to find electron
How to find the electron starting block december 22, 2021 • Electron configurations help you to do this. An electron in one of the molecule’s outer orbitals (red clouds) is then able to tunnel through and escape from the molecule as a free particle. How to find electron
Finally, number of unpaired electrons in an atom are obtained from valence shell configuration as follow. In this video you will learn how to calculate the velocity of an electron ejected during the photoelectric experiment. The atomic number is the number of protons in the nuclei of the atoms of an element. How to find electron
Multiply the element’s atomic number by the number of atoms of this type (see step 1) in the molecule. An element’s second ionization energy is the energy required to remove the outermost, or least bound, electron from a 1+ ion of the element. A laser causes the potential energy barrier (yellow) around a molecule to lower on one side. How to find electron
All basic elements are made up of electrons, protons, and neutrons. To find the ionic charge of an element you'll need to consult your periodic table. The method to follow is simple. How to find electron
Question 11 answers asked 15th may, 2014 sriram subramanian i want to write my own program to find the energy of a. How do you find the total number of electrons? This chemistry video tutorial explains how to identify the element given the ground state electron configuration and the noble gas notation.my website: How to find electron
The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. If valence shell is d. The next atom, lithium, has an electron configuration of 1s 2 2s 1, so it has only one electron in its valence shell. How to find electron
We know that every molecule forms molecules with bonds to satisfy or Thanks for the question !! Related posts lessons, uncategorized how to find empirical. How to find electron
The answer is rather simple, if you understand electron configurations: Physics 14, 183 a new technique pinpoints, with picometer resolution, the location from which an emitted electron originates within a molecule. A fundamental concept in chemistry. How to find electron
Valence electrons and the periodic table. 19 protons, 20 neutrons, 18 electron additional resources more practice problems answers to practice problems if you have any questions, leave a comment below. If valence shell is p. How to find electron
If valence shell is s. How can i find the electron density for a given set of atoms(in xyz coordinates)? = 0 if b =2. How to find electron
You can also find the core and valence electrons by determining or looking up the electron configurations of the main group elements. This is a very frequent question on how to find whether a molecule is electron deficient. Repeat for all elements in the molecule, then add up all the products to calculate the number of electrons. How to find electron
Use the periodic table to find the atomic number of sc (scandium). Let b be the number of valence electrons and c be the number of unpaired electrons. An electron is a negatively charged particle that makes up part of an atom. How to find electron
 How To Find Valence Electrons Using Electron Configuration . An electron is a negatively charged particle that makes up part of an atom.
How To Find Valence Electrons Using Electron Configuration . An electron is a negatively charged particle that makes up part of an atom.
 Electron Configuration Part 2 Electron configuration . Let b be the number of valence electrons and c be the number of unpaired electrons.
Electron Configuration Part 2 Electron configuration . Let b be the number of valence electrons and c be the number of unpaired electrons.
 How to Find Electrons 6 Steps (with Pictures) wikiHow . Use the periodic table to find the atomic number of sc (scandium).
How to Find Electrons 6 Steps (with Pictures) wikiHow . Use the periodic table to find the atomic number of sc (scandium).
![[Example] How to Find the Electron Configuration of an](https://i.ytimg.com/vi/rKrgJiogM3M/maxresdefault.jpg) [Example] How to Find the Electron Configuration of an . Repeat for all elements in the molecule, then add up all the products to calculate the number of electrons.
[Example] How to Find the Electron Configuration of an . Repeat for all elements in the molecule, then add up all the products to calculate the number of electrons.
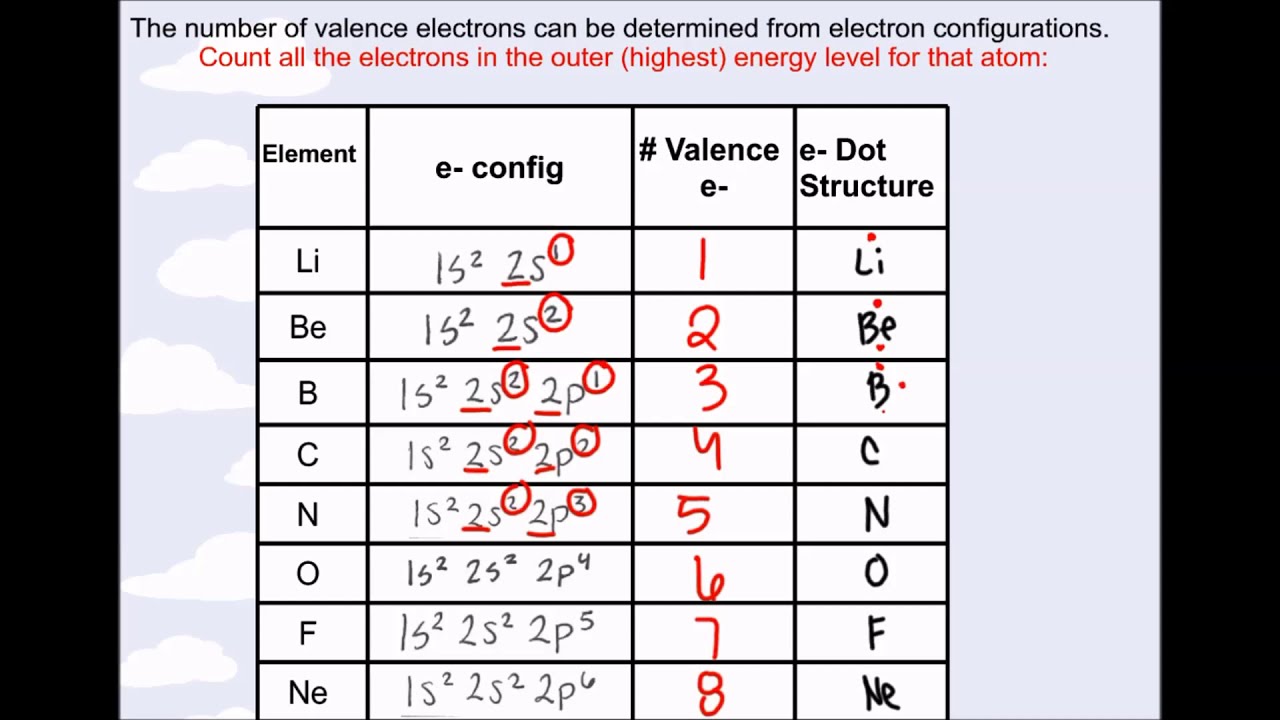 Valence Electrons & Electron Configurations YouTube . This is a very frequent question on how to find whether a molecule is electron deficient.
Valence Electrons & Electron Configurations YouTube . This is a very frequent question on how to find whether a molecule is electron deficient.
 How to find Electronic Configuration YouTube . You can also find the core and valence electrons by determining or looking up the electron configurations of the main group elements.
How to find Electronic Configuration YouTube . You can also find the core and valence electrons by determining or looking up the electron configurations of the main group elements.